An increase in the US’s human parvovirus B19 activity
In brief
This Health Alert Network (HAN) Health Advisory, released by the Centers for Disease Control and Prevention (CDC), informs the public, public health authorities, and healthcare providers about the current surge.
Seasonal respiratory viruses like parvovirus B19 are spread by respiratory droplets from infected individuals who may or may not exhibit symptoms. Public health officials in 14 European nations noted abnormally high numbers of parvovirus B19 illnesses in the first quarter of 2024.
Parvovirus B19 is not routinely monitored in the United States and is not considered a reportable condition. Reports have recently been received by the CDC showing an increase in parvovirus B19 activity in the US. Data include reports of clusters of parvovirus B19-associated problems among pregnant women and sickle cell disease patients, as well as increased test positive for the virus in clinical specimens.
The percentage of individuals with IgM antibodies, a sign of recent infection, rose across all age groups from less than 3% in 2022–2024 to 10% in June 2024; children between the ages of 5 and 9 saw the most increase, going from 15% in 2022–2024 to 40% in June 2024. Pooled samples with parvovirus B19 DNA >104 IU/mL were more common among plasma donors; from 1.5% in December 2023 to 19.9% in June 2024, this percentage rose.
Context
With 50% of vulnerable individuals becoming sick following exposure in the home and 20–50% of susceptible students and staff becoming infected during school outbreaks, parvovirus B19 is extremely contagious through respiratory droplets. Those who work in schools or have frequent contact with children, such as teachers and childcare providers, have historically had a significant occupational risk of infection. By the age of 20, detectable antibodies are present in around 50% of adults. By the time they turn 40, more than 70% of adults have detectable antibodies. It is believed that antibodies from a previous infection can guard against recurrence.
A pregnant woman may contract parvovirus B19 infection from her fetus or from other people through blood component and certain plasma derivative transfusions. The Food and Drug Administration (FDA) advises employing nucleic acid tests to check for parvovirus B19 in all plasma-derived products and plasma units. In the US, whole blood is not parvovirus B19 checked for. It is quite uncommon to contract parvovirus B19 connected with transfusions.
Immunocompetent children and adults who have symptomatic parvovirus B19 infection usually experience a biphasic illness, despite the fact that many infected individuals do not show any symptoms. About seven days after infection, the fever, myalgia, and malaise symptoms define the first phase of the illness.
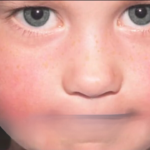
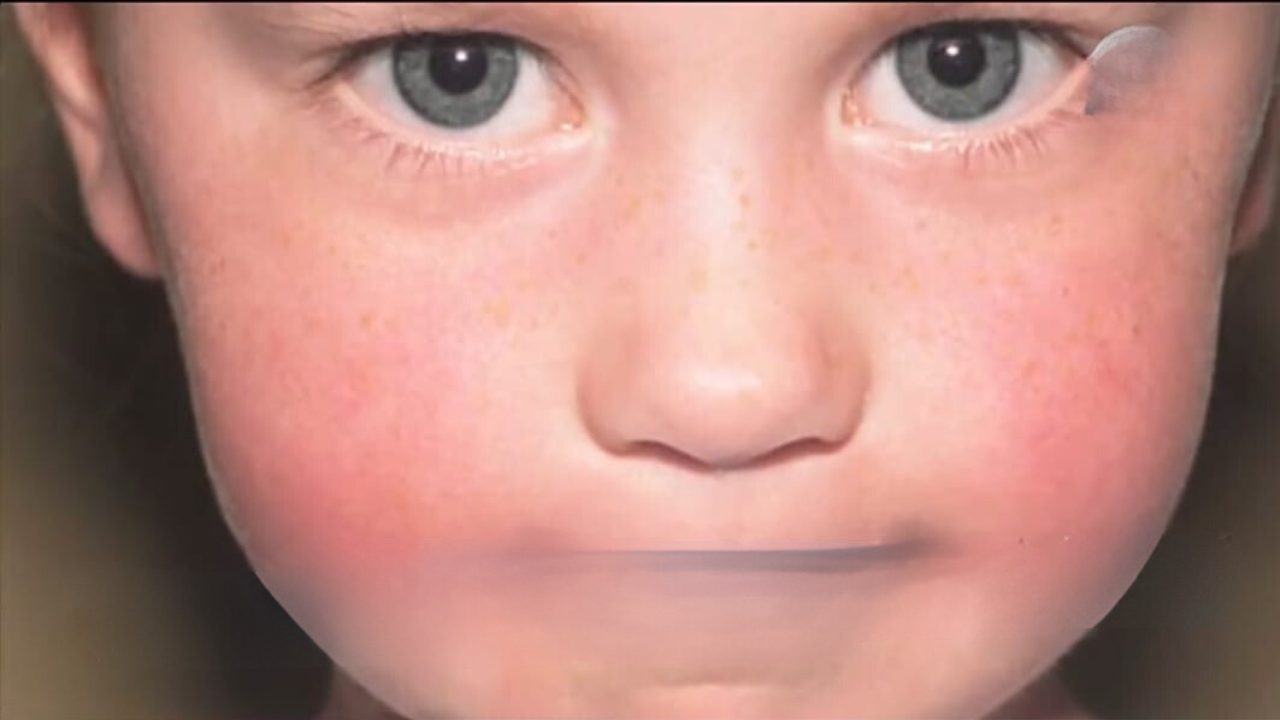
This stage lasts roughly five days. During the first phase of the infection, when viral loads in saliva and respiratory secretions are at their maximum, people with parvovirus B19 are most infectious. Children commonly show with a characteristic facial rash (erythema infectiosum, or “slapped cheek” appearance) during the second phase of the illness, which occurs 7–10 days after the initial phase. 1–4 days later, reticulated body rash or joint pain (arthralgia) may follow.
The most prevalent signs of parvovirus B19 sickness in immunocompetent people are usually a reticular rash on the trunk and joint pain (arthralgia), which usually appear during the second phase of the illness. The distinctive facial rash usually doesn’t show up until after viral loads, which are an indicator of infectiousness, have decreased.
Acute illness-related laboratory testing may reveal leukopenia, moderate anemia, thrombocytopenia, or a brief decline in absolute reticulocyte counts that lasts for around ten days. The majority of patients will recover fully and only need supportive treatment during the acute stage of their disease. Rarely, parvovirus B19 illness might have severe consequences such myocarditis, hepatitis, or encephalitis. For parvovirus B19 infection, neither a vaccination nor a particular course of therapy is advised.
People who are immunocompromised, pregnant, or suffer from chronic hemolytic illnesses and do not have a pre-existing immunity may experience negative health consequences from contracting parvovirus B19 infection. The majority of fetal parvovirus B19 infections during pregnancy end on their own accord and don’t cause any problems.
Nonetheless, there is a 5–10% chance of a poor fetal outcome (such as fetal anemia, non-immune hydrops, or fetal loss), and this risk is highest when acute infection happens between weeks 9–20 of gestation. When a pregnant person has an acute infection, supportive care is provided, and severe fetal anemia is monitored and treated as needed.
In addition, individuals with severe immunocompromising conditions (such as leukemia or other cancers, organ transplants, HIV infection, receiving chemotherapy), as well as those with chronic hemolytic disorders (such as sickle cell disease, thalassemia, hereditary spherocytosis), may experience either transient or chronic aplastic anemia due to parvovirus B19. The two cornerstones of treatment for aplastic anemia are intravenous immunoglobulin and red blood cell transfusions.
Reports have recently been received by the CDC showing an increase in parvovirus B19 activity in the US. These reports provide information from commercial laboratories about rising serological evidence of infection in plasma donors and growing parvovirus B19 test positivity by nucleic acid amplification tests and serology in the general population.
The percentage of individuals with IgM antibodies rose across all age groups from less than 3% in 2022–2024 to 10% in June 2024; children between the ages of 5 and 9 saw the biggest rise, going from 15% in 2022–2024 to 40% in June 2024. Pooled samples with parvovirus B19 DNA >104 IU/mL were more common among plasma donors; from 1.5% in December 2023 to 19.9% in June 2024, this percentage rose.
Suggestions for Medical Professionals
- When someone presents with compatible symptoms (such as fever, rash, arthropathy, or unexplained anemia with low reticulocyte count), be more suspicious of parvovirus B19.
- If someone exhibits compatible symptoms and signs that indicate they may be more susceptible to severe parvovirus B19 disease, provide them preventive counseling and test them at a low threshold. These symptoms and signs include:
a.Those who are expecting
b.People with significantly immunocompromising illnesses, including leukemia or other malignancies, organ transplant, HIV infection, or who are receiving chemotherapy.
c.those suffering from long-term hemolytic blood conditions, such as thalassemia, sickle cell disease, and genetic spherocytosis.
3.Inform patients or their caregivers about high-risk categories and encourage any exposed contacts in those groups—such as those who might be pregnant—to speak with their healthcare professionals while treating patients with suspected or confirmed parvovirus B19.
4.When evaluating pregnant individuals who claim exposure to parvovirus B19 infection or who exhibit compatible signs and symptoms of maternal or fetal parvovirus B19 disease, adhere to standard of care (e.g., professional society standards).
5.Encourage people to follow the CDC’s core preventative methods for preventing respiratory illnesses, such as washing your hands frequently and creating a cleaner environment to stop the transmission of respiratory viruses like parvovirus B19.
a.When working in environments where there is a higher chance of exposure to parvovirus B19, individuals who are more vulnerable to severe consequences or outcomes should wash their hands frequently, refrain from sharing food or beverages, and think about donning a mask or respirator. Removing someone from a workplace where there is a higher chance of exposure to parvovirus B19 has not been shown to be beneficial.
6.In hospital settings, abide by the advised infection control measures for individuals infected with parvovirus B19.
Suggestions for Health Authorities
- Make sure medical professionals are aware of the rising parvovirus B19 activity and can recognize those who are more likely to experience severe parvovirus B19 consequences. There is no need to report parvovirus B19 nationally.
- Encourage the use of preventative measures for respiratory illnesses and tell those who are at a higher risk of developing severe sickness about the implications of parvovirus B19.
- Inform daycare and school providers about the prevalence of parvovirus B19, who may be more susceptible to severe cases, and when staff and students can return to class after contracting the virus.
Advice for the General Public
1.Find out what symptoms the parvovirus B19 causes and who might be more susceptible to a serious illness.
2.Consult a physician if you:
a.are expecting and know someone who has been exposed to someone who may have been infected with parvovirus B19, or you yourself exhibit symptoms similar to parvovirus B19.
b.possess a compromised immune system or a long-term hemolytic blood condition, such as sickle cell disease, thalassemia, or hereditary spherocytosis, together with parvovirus B19 symptoms and indications.
4.To stop the transmission of respiratory viruses, such as parvovirus B19, take general respiratory precautions. Individuals who are more susceptible to severe parvovirus B19 may want to think about utilizing additional preventative techniques, like mask wearing when socializing.
5.Be aware that once the characteristic face rash occurs, people infected with parvovirus B19, including adults and children, are no longer contagious.